Over 30 Years of Experience
Covid-19
Member of the American Academy of Family Physicians | Se Habla Español
Covid-19 Testing
Call us to schedule your appointment (920) 982-7900.
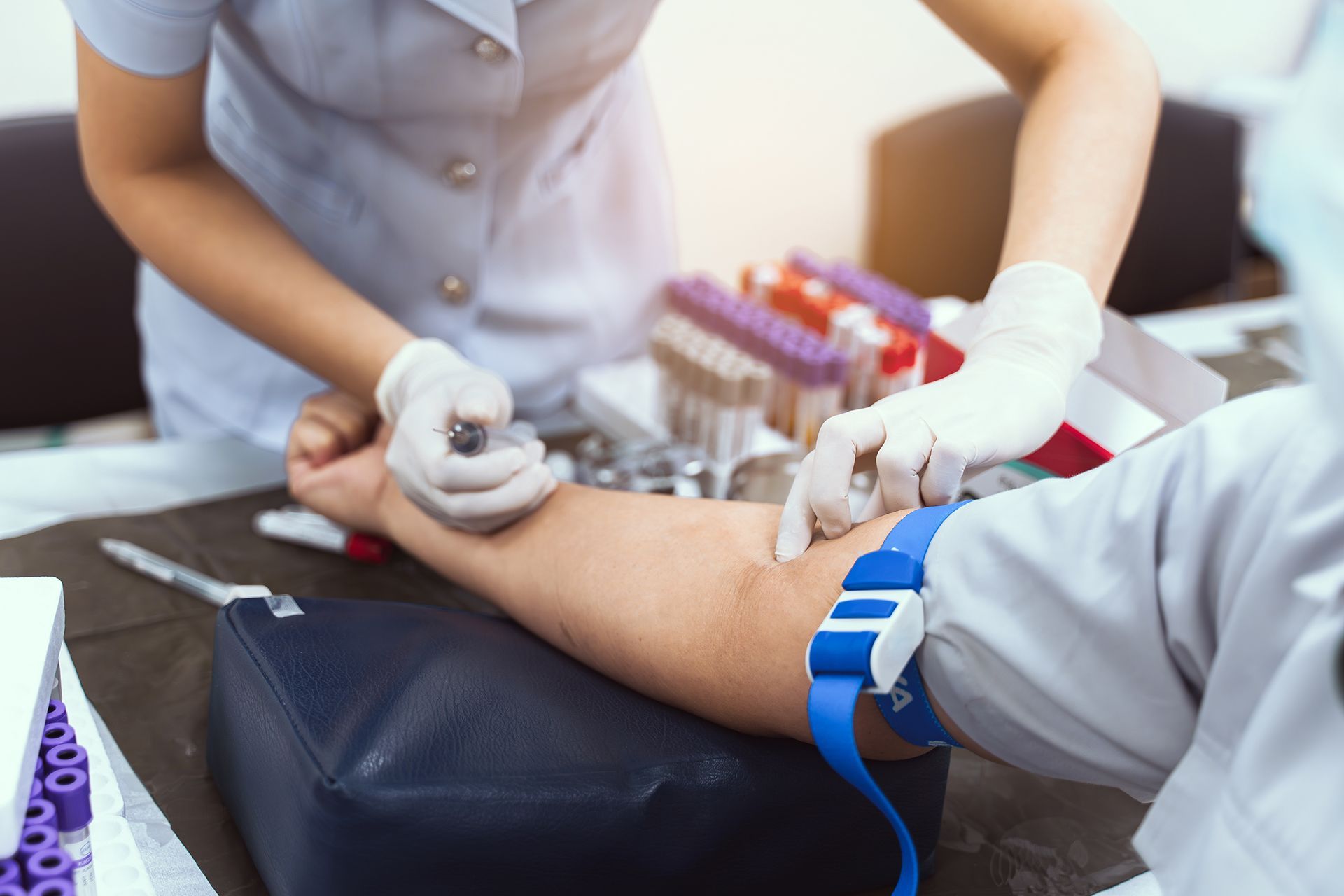
We Now Offer Rapid Testing for Covid-19
VFM and Covid-19 Testing
A novel coronavirus is a new coronavirus that hasn't been previously identified. The virus causing coronavirus disease 2019 (COVID-19), is not the same as coronaviruses that commonly circulate among humans causing mild illnesses, like the common cold.
Valley Family Medicine now has the ability to test patients for active Covid-19 disease (Specifically SARS-CoVIg G) and for Immunity to Covid-19 (Specifically SARS-CoVIg G). Both of these tests are usually done within 2 business days. Call us to schedule an appointment for testing.
Covid-19 Testing for Active Disease
This is a qualitative PCR test for the detection of nucleic acid from patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in nasopharyngeal swabs (NPS) from individuals suspected of COVID-19 infection by their healthcare provider.
Patients with COVID-19 may be asymptomatic or have mild to severe respiratory illness with symptoms of fever, cough, and shortness of breath.
The dry swab direct lysis test is a high throughput nucleic acid methodology that provides results in 24-48 hours of specimen arrival in lab. The current Corona virus PCR tests have been approved for use by regulatory authorities including the FDA and CDC.
These tests are highly specific (no false-positives) and are considered to be very sensitive, approaching 100%, during symptomatic disease when high titers of viral target are present, including directly before symptoms appear and following symptom resolution for several days.
Analytic sensitivity of these assays was determined to be <5 copies of viral genomic RNA per microliter of sample, and the assay has been validated against a panel of specimens provided by the Wisconsin State Lab of Hygiene. Appropriate collection technique is a critical variable for best test performance.
Covid-19 Serology Testing for Immunity
This test is useful to test for IgG antibodies against the SARS-CoV-2 virus, which causes Covid-19 disease.
Test information: This test is reported qualitatively as "Negative", "Indeterminate", or "Positive".
Negative results do not rule out a current SARS-CoV-2 infection, particularly in those who have recently been exposed to the virus. Follow-up testing with a molecular diagnostic test should be considered to rule out infection in these individuals.
Indeterminate results indicate some reactivity of the individual's blood to the components of this test, but serological status cannot be determined. Retesting in 1-2 weeks may be considered.
Positive results may be due to past or present infection with SARS-CoV-2 or non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. Cross reactions with antibodies within the genus Betacoronavirus are known.
If symptoms are present and of recent onset (14 days or less), consider testing with a molecular diagnostic to confirm recent infection.
Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
It is Important to Continue Taking Care of Your Health and Wellness
- Continue your medications, and do not change your treatment plan without talking to your healthcare provider.
- Continue to manage your disease the way your healthcare provider has told you.
- Have at least a 2-week supply of all prescription and non-prescription medications.
- Talk to your healthcare provider about whether your vaccinations are up-to-date.
- Call your healthcare provider
- If you have any concerns about your medical conditions, or if you get sick.
- To find out about different ways you can connect with your healthcare provider for chronic disease management or other conditions. - Do not delay getting emergency care for your health problems or any health condition that requires immediate attention.
- If you need emergency help, call 911.
- Emergency departments have infection prevention plans to protect you from getting COVID-19 if you need care for your medical condition.
- Continue to practice everyday prevention. Wash your hands often, avoid close contact, wear a cloth face covering, cover coughs and sneezes, and clean and disinfect frequently touched surfaces often.